COVID-19, An o2h ventures Perspective
Firstly, we would like to express our sympathies to the families of the 2,400 people who have now died from COVID-19(1). The markets are currently in turmoil regarding the potential economic impact and governments are hurriedly taking radical steps to curtail the spread of the virus. We hope that the biotech and pharma industry can play its part in developing a vaccine or drug as quickly as possible.
In the UK, we have stellar academic and biotech groups that have or are developing breakthrough vaccines and so we thought we would seek expert opinions on how quickly a vaccine could be developed.
Dr Eddy Littler, COO at ReViral is an expert in virology and has a track record in the development of antiviral therapeutics. ReViral currently has a vaccine for RSV in Phase II clinical trials.
“The identification of a new human coronavirus COVID-19 has stimulated an interest in identifying antivirals or vaccines. Clinical trials have started in China to test off-the-shelf inhibitors the most promising of which is the Gilead drug Sovaldi which has been shown previously to be active against both Ebola and SARS in the laboratory. Several companies are screening their compound collections against coronaviruses to identify new leads however even with accelerated approval these new agents will take several years (>4) to be demonstrated to be safe and effective in the clinic. The development of a safe and effective vaccine for COVID-19 would be highly attractive however research has only started to identify protective antigens; select suitable methods of delivery and to show the vaccine to be safe and effective. We should all remember that respiratory syncytial virus is responsible for the death of over 200,000 babies and adults each year and yet despite efforts to develop a vaccine for over 30 years, to date, there is no vaccine that has been shown to be effective beyond phase 2 clinical studies.”
We also managed to get an opinion from Neil French, a Professor of Infectious Diseases and Global Health and Hon Consultant Infectious Diseases Royal Liverpool & Broadgreen University Hospitals Trust.
“It is possible to develop a vaccine ready for GMP manufacture in approx. 4 months, with 6-12 months being a more realistic timescale. However there is a high risk that the vaccine will not be sufficiently immunogenic and multiple parallel designs could be pursued to mitigate this, which increases costs. How long to get such a vaccine to market? If you have a proven platform and construct pH1N1 influenza vaccines were ready in 12 months, but look at Ebola as a wholly novel pathogen the answer is several years and this is more likely for COVID-19.”
We also asked Graham Richards, a former professor at Oxford University and the Chairman and Founder of Oxford Drug Design his views which he succinctly summarised in one line “To find one, months, but to get into use, at least a year.”
These are of course just three professional opinions, but it does give an indication that this may not be as quick to develop a vaccine for COVID-19 as the 3-9 months I have read in various media reports and reality is that we could be several years away before we find an efficacious treatment. We hope a thriving UK biotech industry can contribute to this human health scare.
Biotech SPDR Index outperforming the S&P 500
The macro environment for Biotech remains positive, even though the S&P 500 has had a phenomenal run over the last 6 months, the SPDR Biotech Index has outperformed other indices this year, see Figure 1 below (the dark blue line is the SPDR Biotech Index).
FIGURE 1: S&P 500 Vs SPDR BIOTECH INDEX(2)
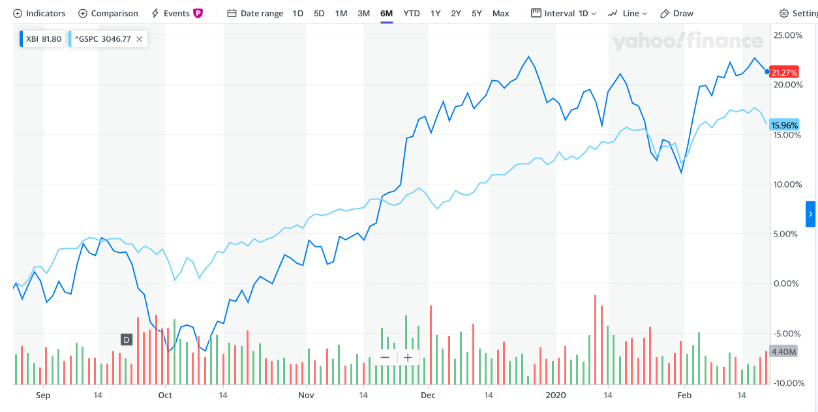
This could be in-part due to the massive acquisition that we have seen in 2019 (Bristol Myers Squibb for Celgene, AbbVie for Allergan, and Amgen for Celgene’s psoriasis drug Otezla—which have together delivered more than $150 billion of value)(3)
The core hypothesis of o2h ventures Fund that the large Pharma will seek to acquire or collaborate with smaller innovative biotech companies – is firmly being played out with this kind of deal flow. Exonate, an o2h ventures Fund investment collaboration with the world’s largest pharma Johnson and Johnson is a homegrown example of this hypothesis (please see – Exonate and J&J Collaboration).
UK Biotech Rockstars!
I have heard many times that we don’t have the management talent that the Americans have in leading our UK biotech companies. Whilst I agree that the talent in the USA is fantastic, we have equally great talent in the UK and we are backing them! I recently conducted short interviews with three of our UK Biotech Rockstars with more interviews to follow..! Watch this space…
Dr Catherine Beech, CEO of Exonate
Dr Chris Torrance, CEO of Phoremost
Mr Robert Boyle, CEO of Sentinel Oncology
Recently Completed Investments
We are very busy closing new investments before the end of the tax year. In the last 12 months o2h ventures EIS Fund have invested into 10 companies targeting a broad range of therapeutic areas: Ocular, Antibiotics, Oncology, Stroke, Rare Diseases, Depression, and Inflammation.
About the o2h ventures Biotech Therapeutics S/EIS Fund
- Prashant and Sunil are the co-founders of o2h ventures. They have been entrepreneurs within the UK biotech industry since 2005, and have had 3 exits that have returned ~10X to their name. They have invested 10% of the total capital deployed from the Fund into investee companies to date.
- o2h ventures has launched Britain’s first EIS and or SEIS investment Fund backing biotech therapeutic and related AI opportunities with plug-in capabilities in Cambridge, UK/India and a bridge to the USA.
Sunil Shah, Fund Manager, the o2h therapeutics and AI S/EIS fund
- The o2h ventures fund is structured as an S/EIS Fund. UK tax payers have the potential to gain through various tax reliefs (Income, Loss, CGT and IHT), The British Government’s best kept secret!
- Investors have the potential to claim tax back for the year 2019/2020 through the carry-back rule for funds deployed before April 5th 2020. o2h ventures has several investments which are oven ready and we are able to deploy funds in this tax year. Cleared funds must be received before March 15th 2020.
- o2h ventures manages risk by investing your Subscription into a portfolio of a minimum of 5 investments. Within this portfolio the companies have business models spread across three buckets: 1. Licensing and Royalties (typical of developing a therapeutic asset) 2. Fee for service/product sales (companies providing a service or product to the industry) 3. Platform fee and milestones (seen more typically with the AI / ML platforms).
- Fund is evergreen with quarterly closes, the next close is on 15th March 2020. To download the IM and onboard, please visit www.o2hventures.com
o2h ventures Limited is regulated and authorised by the Financial Conduct Authority (FRN 812245). Capital at risk, only suitable for high net worth and sophisticated investors.
Notes

